The Definitive Guide to Launching an FDA-Registered Medical Device
1. Introduction: The High-Stakes World of Medical Device Launches
Launching a medical device in the United States isn’t like releasing a new fitness gadget or a productivity app.
It’s not just about engineering, branding, or building a sales funnel — it’s about navigating one of the most complex, high-stakes regulatory environments in the world.
Your device won’t even touch a patient’s hands until you’ve cleared a maze of FDA rules, testing requirements, and compliance systems that can feel like a full-time job in themselves. And here’s the kicker: every day of delay costs money, erodes competitive advantage, and can give a rival the opening they need to beat you to market.
Yet despite the complexity, the reward is enormous. The U.S. medical device market alone is projected to hit $240 billion by 2030 (Fortune Business Insights, 2024), driven by aging populations, chronic disease management, and advances in AI-powered diagnostics. A successful launch not only gives you a slice of that pie — it can position your company for acquisition, global expansion, or even IPO.
The FDA’s role is to ensure devices are safe, effective, and appropriately marketed. That’s non-negotiable. But understanding how the FDA thinks, which submission pathway fits your product, and what you need to prepare long before submission day is where companies either set themselves up for success — or dig their own grave.
Why “FDA-Registered” Matters
The term “FDA-registered” isn’t just marketing fluff. In the eyes of hospitals, procurement officers, and healthcare buyers, it signals legitimacy. Without registration:
- You can’t legally sell your device in the U.S.
- You can’t market it for patient use.
- You risk enforcement actions, product seizures, and crippling fines.
Many founders misunderstand the difference between:
- FDA Registration – Required for all medical device manufacturers, foreign and domestic. It’s essentially letting the FDA know your facility and devices exist, along with paying the annual establishment registration fee.
- FDA Clearance/Approval – A formal review process (via 510(k), PMA, or De Novo) to prove your device is safe and effective for its intended use.
You can be registered without being cleared or approved — but you cannot market your device for patient use until the clearance/approval step is complete.
The Hidden Economics of Device Launches
Here’s what many first-time founders underestimate:
- Regulatory costs often rival R&D costs.
- The time-to-market can be anywhere from 9 months to 3+ years depending on device classification and clinical data requirements.
- A missed compliance detail can add 6–12 months of delay and burn through millions in overhead.
For example:
- Class II devices going through a 510(k) pathway might cost $75k–$250k in regulatory preparation.
- Class III devices needing a PMA can easily exceed $1.5–$5M in total pre-market spend, much of that tied to clinical trials.
Why This Guide Exists
Most “how to launch” articles on medical devices are either:
- Written by lawyers, for lawyers (dense, intimidating, no business focus), or
- Marketing pieces from consultants with little real-world operational experience.
This guide takes a different approach.
It blends:
- Regulatory expertise – so you know exactly what the FDA expects.
- Commercial strategy – so you build a go-to-market plan alongside your compliance roadmap.
- Real-world lessons – from companies that navigated the process successfully (and some that didn’t).
By the end, you’ll know:
- How to classify your device correctly (critical for avoiding rejections).
- Which FDA submission pathway matches your product and market goals.
- How to prepare your documentation, testing, and quality systems in the right order.
- How to align your regulatory process with your product launch, so you’re not burning cash in dead space.
This isn’t theory. This is the playbook I’ve used and seen used in the field — and it’s built for founders, CMOs, product managers, and regulatory teams that need to move fast without cutting corners.
2. Understanding FDA Device Classification
If the FDA is the gatekeeper to the U.S. medical device market, classification is the map that tells you which gate you’re approaching — and how hard it will be to get through.
Get this step wrong, and you can spend months (or years) preparing the wrong documentation, only to have the FDA reject it and send you back to square one.
The Three Classes of Medical Devices
The FDA uses a risk-based classification system for medical devices, defined in the Federal Food, Drug, and Cosmetic Act (FD&C Act).
Class I: Low-Risk Devices
- Examples: Tongue depressors, stethoscopes, bandages, surgical gloves.
- Regulatory Path: Most Class I devices are exempt from premarket notification (510(k)), meaning you don’t need to submit clinical data before selling — but you dostill need to register your establishment and list your device with the FDA.
- Requirements:
- Follow General Controls (proper labeling, manufacturing under a quality system, device listing, and registration).
- Maintain records for potential inspections.
- Common Mistake: Assuming “low risk” means “no oversight.” Even exempt devices can be pulled if labeling or quality control is inadequate.
Class II: Moderate-Risk Devices
- Examples: Blood pressure cuffs, powered wheelchairs, contact lenses, pregnancy test kits.
- Regulatory Path: Most Class II devices require a 510(k) premarket notification — you must demonstrate that your device is substantially equivalent to an existing legally marketed device (predicate device).
- Requirements:
- General Controls plus Special Controls (performance standards, post-market surveillance, patient registries).
- Usually some form of bench or clinical testing data.
- Common Mistake: Underestimating the amount of testing required. Many Class II devices require significant verification and validation work before submission.
Class III: High-Risk Devices
- Examples: Implantable pacemakers, cochlear implants, heart valves.
- Regulatory Path: Almost always requires Premarket Approval (PMA) — the FDA’s most rigorous review process.
- Requirements:
- General + Special Controls.
- Extensive clinical trial data to prove safety and effectiveness.
- Longer review timelines (often 1–3 years).
- Common Mistake: Not budgeting for the full PMA process. Class III submissions are costly, data-heavy, and unforgiving of errors.
Exemptions & Special Classifications
- 510(k)-Exempt Devices: Many Class I and some Class II devices don’t require a 510(k) if they meet specific criteria. The FDA maintains an online database of exempt devices.
- De Novo Classification: If your device is novel but low- to moderate-risk with no predicate device, you can request a De Novo classification. This creates a new device type in the FDA database, opening the door for future devices to use it as a predicate.
- Combination Products: If your device includes drugs or biologics (e.g., a drug-eluting stent), you’ll face a more complex review process involving multiple FDA centers (CDRH + CDER/CBER).
How to Determine Your Device’s Classification
- Search the FDA Product Classification Database — Look up devices with similar intended uses.
- Check Predicate Devices — For Class II devices, identify products already cleared via 510(k) that match your intended use and technological characteristics.
- Review Guidance Documents — The FDA publishes device-specific guidance that outlines applicable regulations and testing requirements.
- Request a 513(g) Letter — For $5,803 (small businesses: $2,902), you can formally ask the FDA how your device will be classified.
- Engage a Regulatory Consultant — Especially valuable if your device falls into a gray area between classes.
Pro Tip: Align Classification with Market Strategy
Your classification dictates time-to-market, regulatory costs, and testing burden.
- If speed is critical, aim for a Class II with a clear predicate device.
- If your device is novel but lower-risk, consider a De Novo strategy — you’ll blaze the trail, but it could be a competitive moat.
- If you’re Class III, start clinical trial planning early and align with reimbursement strategy — you’ll need both to make the numbers work.
3. Navigating FDA Regulatory Pathways
Once you know your device classification, the next step is to choose the correct regulatory pathway to get your product legally marketed in the U.S.
This is where companies often hit delays — not because the product isn’t good, but because they chose the wrong pathway or submitted incomplete documentation.
The Four Main FDA Regulatory Pathways
A. Premarket Notification – 510(k)
Best For: Class II devices (and some Class I) that are substantially equivalent to an existing legally marketed device (predicate).
Goal: Show that your device is as safe and effective as the predicate device in terms of intended use and technological characteristics.
Timeline: ~90 FDA review days (real-world average: 5–9 months including back-and-forth).
Cost:
- Standard FDA fee (2024): $21,760
- Small Business fee: $5,440
Key Steps:
- Identify a Predicate Device: Search the FDA database for a device with the same intended use.
- Conduct Comparative Testing: Bench, lab, and sometimes clinical data to prove equivalence.
- Prepare the 510(k) Submission: Include device description, labeling, test results, risk analysis, and predicate comparison.
- Respond to FDA Requests for Additional Information: Be ready for Q&A cycles.
Pro Tips:
- Choose a predicate cleared recently — older predicates may not meet current standards.
- If your tech is significantly different, the FDA may reject your equivalence claim, pushing you toward a De Novo or PMA.
B. Premarket Approval – PMA
Best For: Class III, high-risk devices with no predicate device — where safety and effectiveness must be proven through clinical trials.
Goal: Provide valid scientific evidence through well-controlled studies to show your device’s benefits outweigh its risks.
Timeline: FDA review goal of 180 days, but real-world timelines often extend to 1–3 years due to trial length and review cycles.
Cost:
- Standard FDA fee (2024): $483,560
- Small Business fee: $120,890
Key Steps:
- Pre-Submission Meeting: Get FDA feedback on trial design before starting.
- Conduct Clinical Trials: Must comply with Good Clinical Practice (GCP).
- Compile PMA Application: Includes full technical, manufacturing, and clinical data.
- FDA Panel Review: Often involves an advisory committee for high-profile devices.
Pro Tips:
- Budget for millions in trial costs.
- Build your reimbursement strategy in parallel — PMA devices often require payer adoption work before launch.
C. De Novo Classification
Best For: Novel, low- to moderate-risk devices without a predicate device.
Goal: Create a brand-new classification regulation for your device type.
Timeline: FDA goal is 150 days; real-world averages are 8–12 months.
Cost:
- Standard FDA fee (2024): $151,470
- Small Business fee: $37,868
Key Steps:
- Prepare a De Novo Request: Show that general and special controls can ensure safety and effectiveness.
- Provide Supporting Data: Bench, animal, or limited clinical testing.
- Await FDA’s Final Order: If granted, your device becomes a predicate for future devices.
Pro Tips:
- This can be a competitive moat — you own the first predicate in your category.
- Consider filing as a “Direct De Novo” if you’re confident no predicate exists, to save time vs. a rejected 510(k).
D. Exempt Devices
Best For: Low-risk Class I and some Class II devices.
Goal: Register and list your device without a formal premarket submission.
Timeline: Weeks — as fast as you can complete your registration, listing, and labeling compliance.
Cost:
- Establishment Registration fee (2024): $7,653
- No user fee for the device itself.
Key Steps:
- Confirm Exemption Status: Check FDA’s device exemption database.
- Register Establishment & List Device: Renew annually.
- Maintain Compliance: Quality system, labeling, and reporting obligations still apply.
Pro Tips:
- Even exempt devices can be recalled — don’t skip risk analysis or quality documentation.
Choosing the Right Pathway
Here’s a quick reference table for decision-making:
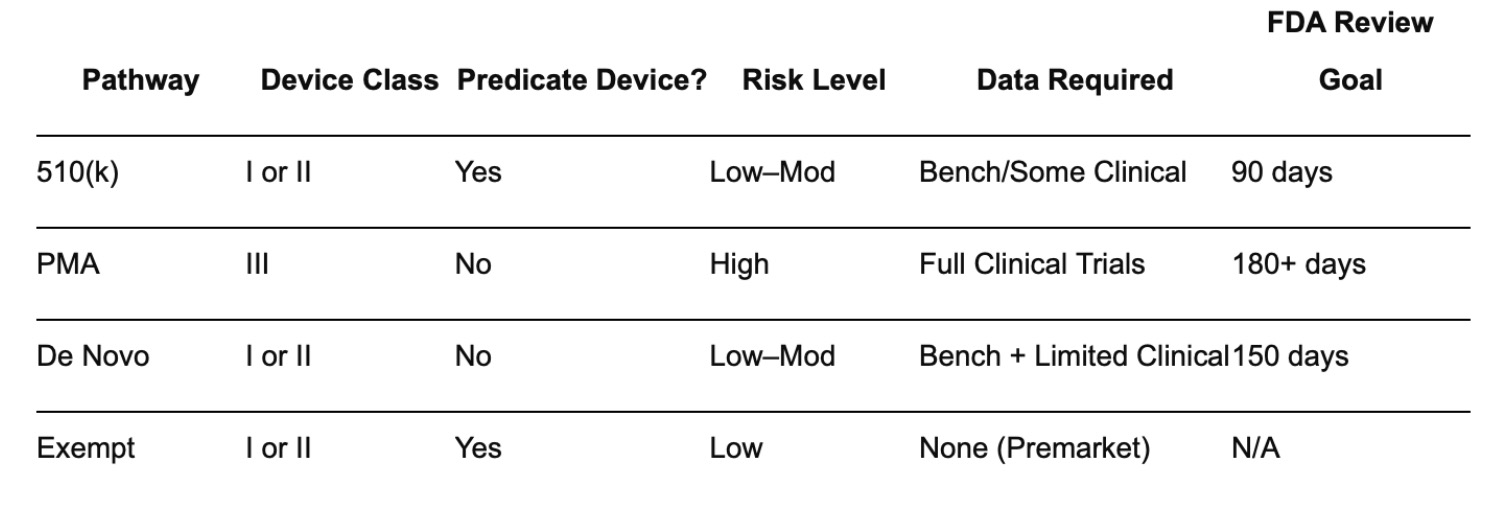
4. Pre-Submission Strategy & Early FDA Engagement
If classification tells you what your device is in the FDA’s eyes, your pre-submission strategy determines how you’re going to get it cleared, approved, or classified — and how fast.
Too many device companies treat FDA interaction as a “final step” when in reality, the most successful launches start talking to the agency before they’ve built the final prototype.
Why Early FDA Engagement is Non-Negotiable
- Clarifies expectations – Avoids wasted time pursuing the wrong data or pathway.
- Identifies red flags – FDA reviewers may point out potential risks or testing requirements you hadn’t considered.
- Establishes rapport – Regulatory affairs is a human process; building goodwill and credibility early can smooth future interactions.
The most common way to engage early is through the Q-Submission Program (Q-Sub) — a voluntary process for requesting FDA feedback before formally submitting your application.
The Q-Sub Process
The Q-Sub program covers several types of meetings, but the most relevant for most companies are:
1. Pre-Submission (Pre-Sub)
- Purpose: Get the FDA’s input on your testing plan, intended use, and regulatory pathway before you invest heavily in trials or validation.
- When to Use: Early in product development, ideally after proof-of-concept but before final verification and validation.
- Format: You submit a written package of questions and supporting documents, then meet (virtually or in person) with the FDA to discuss.
2. Informational Meetings
- Purpose: Share updates or introduce the FDA to novel technologies.
- When to Use: If your device uses emerging tech like AI/ML or has unique manufacturing methods.
3. Submission Issue Meetings
- Purpose: Resolve questions during the review of an already-submitted application.
- When to Use: If the FDA issues a “request for additional information” and you need clarification.
How to Prepare for a Pre-Sub
- Define Your Questions – Be specific. Instead of “What testing do we need?” ask “Will the proposed 12-week bench test be sufficient to demonstrate durability under ISO 10993-5?”
- Provide Enough Context – Include device description, intended use, and a summary of preliminary testing or risk analysis.
- Propose a Pathway – Don’t ask the FDA to decide for you; present your best classification and justification, and invite feedback.
- Bring the Right People – Include your regulatory lead, engineering rep, and clinical advisor if applicable.
- Follow Up in Writing – Document FDA feedback and align your team immediately after the meeting.
Timing Considerations
- FDA Review of Pre-Sub Requests: Usually 70–75 days from request to meeting.
- Plan your development schedule so Pre-Sub feedback arrives before major design freeze or trial initiation.
- A well-timed Pre-Sub can shave 3–6 months off your total time-to-market by preventing rework.
Real-World Example
A mid-sized diagnostics company developing a novel saliva-based cancer screening test submitted their initial 510(k) without engaging in a Pre-Sub. The FDA rejected their equivalence claim because the predicate device used blood samples, not saliva. The company lost 10 months redesigning studies and resubmitting.
Had they used the Q-Sub process, the FDA would have flagged the predicate issue upfront — saving nearly a year.
Pro Tips for Effective Pre-Subs
- Ask “deal-breaker” questions first — anything that could force a pivot.
- Don’t overwhelm with data dumps; focus on the most relevant evidence for your stage.
- Treat the meeting as collaborative, not adversarial. The FDA’s job is to ensure safety — if you help them see how your device achieves that, you both win.
5. Design & Development Under FDA Design Controls
Once you’ve clarified classification and engaged the FDA early, your next focus is design and development under FDA design controls — a regulatory framework that ensures your device is safe, effective, and meets user needs.
For most Class II and Class III devices (and some Class I exempt), compliance with 21 CFR Part 820.30 — the FDA’s design control regulation — is not optional. Even if you’re in a small startup, skipping or loosely documenting these steps can cost you months in rework or trigger a failed inspection.
Why Design Controls Matter
Think of design controls as both a legal requirement and a business insurance policy:
- They force you to define what the product must do before you build it.
- They ensure that the final device can be traced back to original requirements.
- They create a documented “story” the FDA can follow from concept to commercial product.
The Core Elements of FDA Design Controls
Per 21 CFR Part 820.30, here’s what you must have in place:
1. Design & Development Planning
- Document a plan that defines design tasks, responsibilities, and timelines.
- Update the plan as the project evolves — the FDA expects “living documents,” not static PDFs.
2. Design Inputs
- Define measurable requirements your device must meet (performance, safety, usability).
- Include both regulatory requirements (e.g., IEC 60601 for electrical safety) and user-driven needs.
3. Design Outputs
- The actual specifications, drawings, and manufacturing instructions that will be used to produce the device.
- Must be verifiable against design inputs.
4. Design Review
- Conduct formal, documented reviews at key milestones.
- Include cross-functional team members and independent reviewers (not just the design engineer).
5. Design Verification
- Confirm that design outputs meet design inputs — often through lab testing, bench testing, or simulated use.
6. Design Validation
- Confirm that the final device meets user needs and intended uses — often through clinical studies or usability testing in actual use environments.
7. Design Transfer
- Ensure that the device design is correctly translated into production specifications without loss of function or quality.
8. Design Changes
- Maintain a process for reviewing, approving, and documenting any design modifications throughout the lifecycle.
9. Design History File (DHF)
- A complete record of the design process, from initial concept through final design — the FDA will inspect this during audits.
Integrating Usability Engineering
The FDA now expects devices to meet human factors engineering requirements, especially if misuse could lead to harm.
Key steps:
- Conduct user research early.
- Build use-related risk analyses (URRA).
- Run formative usability tests before locking design.
Risk Management (ISO 14971 Alignment)
The FDA harmonizes with ISO 14971 for medical device risk management.
- Identify hazards.
- Estimate and evaluate risks.
- Implement control measures.
- Monitor and update throughout the product lifecycle.
Common Pitfalls
- Treating design controls as paperwork after the fact instead of integrating them from day one.
- Using vague, unmeasurable design inputs (“must be user-friendly”).
- Failing to connect verification and validation activities directly to documented requirements.
Case Study: The $2M Recall That Could Have Been Avoided
A wearable cardiac monitoring startup rushed to market without formal usability validation. Users misunderstood an indicator light, leading to improper device use and false-negative results.
- Outcome: FDA recall, 18 months lost, $2M in remediation costs.
- Lesson: Usability isn’t an afterthought — it’s part of regulatory compliance.
6. Building a Quality Management System (QMS) That Scales
If design controls are the project-specific rules, your Quality Management System (QMS) is the organization-wide rulebook that keeps every product compliant.
For FDA-registered devices, a compliant QMS is not optional — it’s the backbone of your regulatory standing and your ability to scale without chaos.
Why the QMS Matters
The FDA enforces the Quality System Regulation (QSR) under 21 CFR Part 820, which requires manufacturers to establish and maintain a QMS that ensures products consistently meet requirements and are safe for use.
A QMS does three critical things for your business:
- Protects You During Audits — The FDA will inspect your QMS, not just your device.
- Prevents Costly Mistakes — Systematic processes catch issues before they reach patients.
- Enables Faster Scaling — When you add new products or manufacturing partners, the QMS ensures consistency without reinventing the wheel.
Core QMS Components Under 21 CFR Part 820
At a minimum, your QMS must cover:
- Management Responsibility — Clear leadership accountability for quality.
- Quality Audits — Regular internal reviews of QMS effectiveness.
- Personnel Training — Documented training records and competency checks.
- Document Controls — How documents are created, approved, distributed, and revised.
- Purchasing Controls — Vetting and monitoring suppliers to ensure incoming materials meet specs.
- Production & Process Controls — Including process validation and in-process testing.
- Corrective and Preventive Actions (CAPA) — Systematic response to quality issues.
- Complaint Handling — Including Medical Device Reporting (MDR) when required.
- Record Keeping — Device History Records (DHR), Device Master Records (DMR), and Quality Records.
ISO 13485: The Global Benchmark
While the FDA has its own QSR, many companies also align with ISO 13485, the international standard for medical device QMS.
- Benefit 1: Easier global market entry.
- Benefit 2: Future-proofing — the FDA is moving toward greater alignment with ISO 13485 under its proposed QMSR (Quality Management System Regulation).
QMS Implementation Approaches
Option 1: Build from Scratch
- Full control over processes and documentation.
- Best for highly specialized products with unique processes.
- Time-intensive and higher upfront cost.
Option 2: Off-the-Shelf QMS Software
- Solutions like Greenlight Guru, Qualio, and MasterControl offer pre-built templates aligned to FDA and ISO requirements.
- Faster to deploy, especially for startups.
Option 3: Hybrid Approach
- Start with off-the-shelf, customize as you grow.
QMS and Outsourced Manufacturing
If you use a contract manufacturer:
- You still own regulatory responsibility.
- Your QMS must include supplier quality agreements.
- You must conduct supplier audits to verify compliance.
CAPA: Your Continuous Improvement Engine
CAPA (Corrective and Preventive Action) is one of the most critical — and most scrutinized — elements of your QMS.
- Corrective Action: Fixing a problem after it occurs.
- Preventive Action: Eliminating the cause of a potential problem before it happens.
The FDA frequently cites CAPA deficiencies in warning letters — making it a prime audit target.
Common QMS Pitfalls
- Treating the QMS as a binder on a shelf rather than a living system.
- Failing to train all employees on QMS relevance to their role.
- Not linking CAPA outcomes back to risk management.
Case Study: The Startup That Passed FDA Inspection in Year 1
A Class II orthopedic device company implemented Greenlight Guru’s eQMS six months before their first production run.
- Created standardized SOPs for supplier audits, document control, and training.
- Conducted mock FDA audits every quarter.
- Result: Passed their first FDA inspection with zero Form 483 observations.
7. Preclinical & Bench Testing: Proving Safety Before Human Use
Before a medical device can touch a patient, it has to prove — on paper and in the lab — that it’s safe, reliable, and performs as intended.
For FDA-registered devices, preclinical testing is the bridge between your design and your first human use.
The Purpose of Preclinical Testing
Preclinical testing answers two key regulatory questions:
- Is it safe? — The device should not cause harm when used as intended.
- Does it work as intended? — The device should deliver consistent, reliable performance under real-world conditions.
This stage is not optional — skipping or rushing it can cause delays, failed submissions, or even recalls.
Types of Preclinical Testing
Depending on your device classification and intended use, testing may include:
1. Bench Testing
- Simulates real-world use without involving living tissue.
- Examples:
- Mechanical stress tests (durability, load-bearing capacity)
- Fluid flow testing (catheters, infusion pumps)
- Electrical safety and EMC testing (electronic devices)
- Standards often referenced: ASTM International, IEC 60601 (for electrical devices).
2. Biocompatibility Testing
- Required if your device contacts the patient’s body.
- Standard: ISO 10993 series (Biological evaluation of medical devices).
- Tests may include:
- Cytotoxicity (cell damage potential)
- Sensitization (allergic reactions)
- Irritation
- Systemic toxicity
3. Sterility & Shelf-Life Testing
- For sterile devices: verify sterilization method effectiveness (ethylene oxide, gamma radiation, steam).
- Shelf-life studies confirm sterility and performance remain intact over time.
4. Simulated Use Testing
- Mimics how the device will actually be handled and used.
- Helps identify human factors issues before clinical trials.
Aligning Testing with Regulatory Strategy
Not all tests are created equal — the FDA expects testing to be:
- Relevant — Matched to your device’s risk profile.
- Standardized — Based on recognized consensus standards.
- Documented — Complete protocols, raw data, and analysis must be archived.
💡 Tip: Submit a Pre-Submission (Q-Sub) to the FDA to confirm your testing plan before starting expensive protocols.
Common Preclinical Testing Mistakes
- Over-testing — Spending budget on unnecessary tests not required for your device class.
- Under-testing — Missing critical performance or safety validations.
- Poor documentation — Incomplete records can lead to costly retests.
- Ignoring usability — Overlooking human factors can cause post-market issues.
Case Study: Avoiding a $150K Testing Mistake
A startup developing a disposable surgical instrument assumed they needed full biocompatibility testing for every material in the device.
- After consulting with a regulatory expert and referencing ISO 10993, they realized two components were “external communicating devices” with short-term exposure — requiring fewer tests.
- Savings: $150,000 in unnecessary testing costs.
- Time saved: 4 months in their submission timeline.
Key Documentation Outputs
- Test protocols (what you plan to do)
- Test reports (what you actually did and found)
- Summary tables for FDA submission
- Traceability matrix linking design inputs to test results
How Preclinical Testing Fits Into the Larger Picture
- Design Controls feed into Testing Requirements.
- Testing Results feed into your Regulatory Submission.
- Passing preclinical tests sets the stage for clinical trials (if required).
8. Clinical Evaluation & Trials: Proving Safety and Efficacy in Humans
Once your device clears preclinical testing, the next step (for certain device classes) is to generate human clinical evidence.
This stage is where you demonstrate — under real-world medical conditions — that your device is both safe and effective for its intended use.
When Are Clinical Trials Required?
Not all FDA-registered devices require human trials. The decision depends on:
- Device classification
- Class I: Rarely requires clinical data (unless novel or high-risk).
- Class II: May require clinical studies if no predicate exists or risk profile is high.
- Class III: Almost always requires clinical evidence.
- Submission pathway
- 510(k): Often relies on predicate data, but novel features can trigger clinical requirements.
- De Novo: More likely to require some clinical data.
- PMA: Requires comprehensive clinical trials.
- Risk profile — Devices that sustain life, diagnose critical conditions, or contact the central nervous system almost always require trials.
💡 Tip: Use the FDA’s Pre-Submission (Q-Sub) process to confirm whether your device needs clinical trials before investing in them.
Types of Clinical Evaluation
Clinical evaluation can take multiple forms:
1. Literature Review
- For low-risk devices with similar marketed products.
- FDA may accept existing peer-reviewed studies as supporting evidence.
2. Retrospective Data Analysis
- Uses historical patient data from past use (if available and compliant with privacy laws).
3. Prospective Clinical Trials
- Actively recruit and follow patients using your device.
- Required for PMA and some De Novo devices.
Clinical Trial Design Essentials
An effective clinical study must:
- Have a clear objective — Define exactly what you’re measuring (safety endpoints, performance metrics).
- Select the right population — Patients that match the device’s intended use.
- Include control groups (if applicable) — Often required for PMA submissions.
- Follow Good Clinical Practice (GCP) — Compliance with 21 CFR Part 812(Investigational Device Exemptions) and ISO 14155.
Key Clinical Metrics
- Safety endpoints — Adverse events, complications.
- Efficacy endpoints — Accuracy, improvement rates, functional gains.
- Usability outcomes — User error rates, training needs.
- Quality of life measures — Patient-reported outcomes (PROs).
Navigating the IDE Process
If a clinical investigation is required:
- Non-significant risk (NSR) devices — Can often proceed with IRB approval only.
- Significant risk (SR) devices — Require an Investigational Device Exemption (IDE)from the FDA before starting trials.
Case Study: Accelerating PMA Approval
A company developing an implantable cardiac monitoring device used an adaptive trial design — allowing modifications to sample size and endpoints based on interim data.
- Result: Completed pivotal trial in 18 months (vs. industry average 36 months).
- Outcome: PMA approval granted in 24 months total.
Common Clinical Trial Pitfalls
- Poor site selection — Choosing sites without relevant patient volume slows recruitment.
- Inadequate training — Leading to high user error rates.
- Underpowered studies — Too few participants to achieve statistical significance.
- Protocol deviations — Can undermine FDA acceptance.
Best Practices for Clinical Trials
- Partner with Contract Research Organizations (CROs) experienced in your device type.
- Use electronic data capture (EDC) systems to ensure real-time compliance monitoring.
- Build in interim analysis checkpoints to pivot if recruitment or results lag.
Outputs from Clinical Evaluation
- Clinical Study Protocol (CSP)
- Informed Consent Forms (ICFs)
- IRB/EC approvals
- Statistical Analysis Plan (SAP)
- Clinical Study Report (CSR) for FDA submission
9. Regulatory Submission Pathways: 510(k), De Novo, and PMA Explained
Once your design, testing, and clinical evaluation are complete, the next step is getting the FDA’s green light to market your device.
This means choosing the correct submission pathway — a decision that affects your timeline, cost, and approval odds.
The Three Main FDA Submission Pathways
Most medical devices enter the U.S. market through one of three routes:
1. 510(k) Premarket Notification
- Purpose: Demonstrate that your device is substantially equivalent to a legally marketed predicate device.
- Best for: Most Class II devices and some Class I devices.
- Requirements:
- Detailed device description.
- Predicate comparison table (materials, design, intended use).
- Performance testing (bench, animal, clinical if needed).
- Labeling and IFU review.
- Timeline: ~90 days FDA review (actual timeline often 4–6 months with interactive questions).
- Cost: FDA user fee ~$21,760 (2024), with small business reduction available.
- Advantages:
- Faster and cheaper than De Novo or PMA.
- No requirement for large-scale clinical trials unless novel risk profile.
- Limitations:
- Must find a valid predicate — if none exists, you can’t use 510(k).
- Predicate must be cleared, not just registered.
2. De Novo Classification Request
- Purpose: For novel, low-to-moderate risk devices without a valid predicate.
- Best for: Innovative devices that don’t fit existing classification.
- Requirements:
- Comprehensive risk/benefit analysis.
- Performance and safety testing (bench, animal, human).
- Post-market surveillance plan (if applicable).
- Timeline: 150 FDA review days (often 6–10 months real time).
- Cost: FDA user fee ~$132,464 (2024), with small business discount.
- Advantages:
- Creates a new device classification you own (first-mover advantage).
- Lower burden than PMA for lower-risk devices.
- Limitations:
- More data required than 510(k).
- Can be rejected if FDA deems the risk profile too high.
3. Premarket Approval (PMA)
- Purpose: For high-risk Class III devices (life-sustaining, life-supporting, or implantable).
- Best for: Devices with no predicate and significant patient risk.
- Requirements:
- Full clinical trial data.
- Manufacturing facility inspection.
- Complete design and quality documentation.
- Post-approval study plan.
- Timeline: 180 FDA review days (realistically 1–2 years).
- Cost: FDA user fee ~$483,560 (2024), small business discount possible.
- Advantages:
- Highest level of FDA clearance.
- Strong market credibility and IP protection.
- Limitations:
- Most expensive and time-consuming pathway.
How to Choose the Right Pathway
Decision Tree:
- Does a predicate exist?
- Yes → 510(k).
- No → Go to #2.
- Is the device low/moderate risk?
- Yes → De Novo.
- No → PMA.
Submission Success Factors
- Pre-Submission (Q-Sub) Meetings — Let you get FDA feedback before formal filing.
- Clear Testing Data — FDA rejections often stem from unclear or incomplete performance evidence.
- Labeling Accuracy — Misleading claims can trigger delays or denials.
- Regulatory Affairs Partner — Using a consultant or in-house expert dramatically improves submission quality.
Case Study: Avoiding a 6-Month Delay
A startup in digital wound monitoring initially planned a De Novo submission but discovered a similar cleared predicate in an FDA database review.
- Switched to 510(k), avoiding the need for new clinical trials.
- Saved ~$110K in fees and cut approval timeline by 8 months.
10. The FDA Review Process & How to Respond to Deficiency Letters
Submitting your application to the FDA isn’t the finish line — it’s the start of a structured, high-stakes review process.
How you manage the next steps determines whether you sail through to clearance or get stuck in a cycle of delays.
Step-by-Step: What Happens After Submission
1. Administrative Review (Day 1–15)
- FDA checks that your submission is complete and formatted correctly.
- They verify payment of user fees, presence of required forms, and completeness of testing data.
- If anything is missing, your application is placed “on hold” until corrected.
2. Substantive Review (Day 15–90 for 510(k))
- Technical reviewers analyze your bench, animal, and/or clinical data.
- They assess substantial equivalence (510(k)) or risk/benefit (De Novo, PMA).
- Labeling, Instructions for Use (IFU), and marketing claims are scrutinized for accuracy.
3. Interactive Review
- FDA sends interactive review questions during evaluation.
- Response windows are short — sometimes as little as 48–72 hours.
- Delays in answering can reset review timelines or result in rejection.
4. Decision Phase
- FDA issues one of three decisions:
- Substantially Equivalent / Granted — Device cleared or approved.
- Not Substantially Equivalent / Denied — Application rejected.
- Additional Information Needed — Submission placed on hold pending more data.
Deficiency Letters: What They Are and How to Handle Them
A Deficiency Letter (sometimes called an Additional Information Request) outlines gaps or problems in your application.
- Common issues include:
- Incomplete test reports.
- Missing risk analysis.
- Vague or unsubstantiated marketing claims.
- Labeling inconsistencies.
Best Practices for Responding
- Acknowledge Immediately — Confirm receipt and commitment to respond within FDA’s deadline.
- Clarify the Request — If unclear, request a quick call with your FDA reviewer.
- Be Complete, Not Quick — Partial answers often trigger another deficiency letter.
- Document All Changes — Update your submission index so reviewers can find new information easily.
Timeline Impact of Deficiency Letters
- One deficiency letter: Adds 1–3 months to review.
- Multiple letters: Can double your timeline and increase costs significantly.
Pro Tip: Avoid Deficiency Letters in the First Place
- Conduct a mock FDA audit of your submission with a regulatory consultant.
- Pre-submit high-risk or novel testing approaches via the Q-Sub process for feedback before formal review.
Case Study: Cutting Review Time by 40%
A wearable heart monitor company submitted a 510(k) with a Pre-Sub-reviewed testing plan.
- FDA had already seen and agreed with their bench and clinical protocols.
- Result: Cleared in 4 months with zero deficiency letters — well under the industry average of 6–9 months.
11. Post-Market Surveillance: Staying Compliant After Launch
Getting your device cleared or approved is only half the battle.
Once it’s on the market, you enter a continuous compliance phase where the FDA expects you to monitor safety, track performance, and report issues.
This is where many device companies stumble — and where costly recalls or warning letters often start.
Why Post-Market Surveillance Matters
- Protects patient safety and brand trust.
- Ensures compliance with 21 CFR Part 803 (MDR reporting) and 21 CFR Part 820 (QSR).
- Provides real-world performance data for next-generation product development.
Core Post-Market Surveillance Obligations
1. Medical Device Reporting (MDR)
- Mandatory: You must report certain adverse events to the FDA within specific timeframes:
- 30 days — Serious injury or malfunction that could cause harm if it recurred.
- 5 days — Events requiring immediate corrective action.
- Use FDA’s MedWatch Form 3500A for submissions.
2. Complaint Handling
- Maintain a complaint file for all device-related issues.
- Each complaint must be evaluated for MDR reporting criteria.
- Document investigation results and any corrective actions taken.
3. Corrective and Preventive Action (CAPA)
- Investigate root causes of quality issues.
- Implement corrective actions (fix the problem) and preventive actions (stop it from happening again).
- Track CAPA effectiveness over time.
4. Post-Market Clinical Follow-Up (PMCF)
- Required for certain higher-risk devices or those with limited pre-market data.
- May involve ongoing clinical studies or patient registries to monitor long-term performance.
5. Recalls & Field Corrections
- If a defect poses a safety risk, you may need to initiate a voluntary recall or field correction.
- Must be reported to the FDA within 10 working days of initiation.
Proactive Surveillance Tools
- UDI Tracking — Unique Device Identification for traceability.
- Customer Feedback Systems — Use digital platforms to capture and categorize feedback in real time.
- Remote Monitoring Integration — For connected devices, pull usage and performance data directly from the field.
Penalties for Non-Compliance
- FDA Warning Letters (public and damaging).
- Seizure of products.
- Civil money penalties (up to $500,000 per violation).
- Consent decrees restricting future operations.
Case Study: Avoiding a Costly Recall
A startup selling a Class II surgical device implemented a real-time digital complaint intake system.
- Caught a recurring handle defect in 3 units before widespread distribution.
- Issued a field correction instead of a recall.
- Saved an estimated $1.2M in recall costs and avoided negative press.
Pro Tip: Treat Post-Market Surveillance as a Competitive Advantage
- Use your surveillance data to prove product reliability in sales pitches.
- Share safety stats and improvement actions in annual reports to build trust with healthcare buyers.
12. Labeling, Marketing, and FDA Enforcement Risks
Once your medical device is on the market, everything you say about it—from the box label to your Facebook ads—falls under FDA scrutiny.
If your claims go beyond what was cleared or approved, you risk warning letters, fines, and even forced removal from the market.
**What Counts as “Labeling” in FDA Terms?
It’s broader than most think:**
- Physical packaging (box, pouch, inserts).
- Instructions for Use (IFU).
- Product website and downloadable PDFs.
- Marketing brochures, trade show materials, and sales decks.
- Digital ads, social media posts, and videos.
If you put it in writing or say it in a way that promotes the product, the FDA can consider it labeling or advertising.
Key Compliance Rules
1. Stick to Cleared or Approved Claims
- FDA clearance or approval covers specific indications for use—don’t imply broader benefits.
- Example: If your Class II wound dressing is cleared for minor cuts and abrasions, you can’t market it as “effective for third-degree burns” without additional clearance.
2. Avoid “Off-Label” Promotion
- Off-label use happens when a device is used in a way not approved by the FDA.
- While doctors may legally use devices off-label, manufacturers cannot promote or market for those uses.
3. Substantiation is Non-Negotiable
- Every claim must be truthful, not misleading, and backed by evidence.
- Keep scientific studies, bench testing results, or clinical trial data on file.
4. Watch Implied Claims
- Even without saying it directly, imagery or context can imply performance beyond clearance.
- Example: Showing a Class II fitness tracker in an ICU could imply it’s a hospital-grade monitoring device.
Enforcement Examples
- Misbranding: FDA warning letter to a dental device company for claiming “instant cavity reversal” without supporting clinical data.
- Unsubstantiated Performance: $3M settlement with FTC for a wearable device marketed as “guaranteeing weight loss.”
- Off-Label Marketing: FDA fines to a surgical device maker for promoting pediatric use without clearance.
Best Practices to Stay Compliant
- Maintain a Marketing Review Process — Have regulatory affairs sign off on all content before release.
- Train Your Sales & Marketing Teams — They must know what they can and can’t say.
- Use Approved Claims Creatively — You can still differentiate with user experience, design features, or customer service—not just clinical claims.
- Monitor Third-Party Marketing — Distributors and resellers can trigger FDA enforcement if they misrepresent your product.
Pro Tip: Your Marketing is a Compliance Asset
- Strong, compliant messaging builds trust with buyers, investors, and regulators.
- Create a claims library—a master list of approved phrases, stats, and images your team can use safely.
13. International Expansion: CE Marking, MDR, and Beyond
Launching in the U.S. is just the start.
If you want to tap into Europe’s $150+ billion medical device market or emerging markets in Asia-Pacific and Latin America, you’ll need to navigate a whole new set of regulations.
Why International Expansion Matters
- Market Diversification — Reduces dependency on U.S. sales.
- Brand Authority — International presence increases valuation and trust.
- Volume Leverage — Higher production runs can reduce per-unit manufacturing costs.
The European Union: CE Marking Under MDR
Step 1: Understand MDR
The Medical Device Regulation (MDR 2017/745) replaced the older MDD framework.
It’s stricter, with higher clinical evidence requirements and expanded post-market surveillance rules.
Key MDR Changes:
- More rigorous clinical data requirements—even for legacy devices.
- Unique Device Identification (UDI) requirements similar to the U.S. system.
- Expanded scope to include some aesthetic devices (e.g., colored contact lenses).
Step 2: Classification & Conformity Assessment
- Devices are classified into Class I, IIa, IIb, III (similar to FDA’s Class I–III, but not identical).
- Most devices require assessment by a Notified Body—independent organizations designated by EU member states to certify conformity.
Step 3: Technical Documentation
- Device description & specifications.
- Risk management files.
- Clinical evaluation report.
- Manufacturing process details.
- Post-market surveillance plan.
Step 4: Affixing the CE Mark
Once certified, the CE mark allows free sale across all EU member states.
You must also register the device in EUDAMED, the EU’s central medical device database.
Canada: Health Canada Licensing
- Class I devices only require manufacturer registration.
- Class II–IV devices need a Medical Device License (MDL) from Health Canada.
- Must comply with ISO 13485 quality management standards.
Asia-Pacific Markets
- Australia: Follows a risk-based classification system under the Therapeutic Goods Administration (TGA).
- Japan: PMDA approval required; often more data-intensive than FDA submissions.
- China: NMPA registration is notoriously slow; requires local clinical trials for many devices.
Latin America
- Brazil: ANVISA approval; timelines vary from 6–18 months.
- Mexico: COFEPRIS regulates; some U.S. FDA clearances can streamline approval.
Common Global Expansion Pitfalls
- Assuming FDA Approval = Global Approval — It doesn’t. Each country has its own process.
- Underestimating Translation Needs — MDR and other regulators require local language labeling.
- Ignoring Local Distribution Laws — Some countries mandate using a local representative.
- Not Planning for Customs & Logistics — Delays at ports can ruin product launches.
Pro Tips for Global Device Launches
- Hire Local Regulatory Experts — Avoid costly mistakes by working with someone who knows the market.
- Leverage Mutual Recognition Agreements — Some countries accept data from other markets to speed approval.
- Plan for Currency and Taxation — Local pricing strategy can make or break profitability.
- Stage Your Expansion — Don’t launch in 10 countries at once; start with 1–3 strategic targets.
14. Post-Market Surveillance, Recalls, and Ongoing Compliance
Getting FDA clearance or CE marking is only the start — once your device hits the market, the real work of maintaining compliance begins.
Post-market surveillance (PMS) is not optional; it’s a regulatory requirement in most jurisdictions and a critical safeguard for your brand.
Why Post-Market Surveillance Matters
- Regulatory Obligation — FDA, EU MDR, and other agencies require ongoing monitoring.
- Risk Management — Detect problems early before they escalate into safety crises.
- Brand Protection — Transparency in handling issues builds trust with customers and investors.
- Competitive Advantage — Feedback loops from PMS can fuel product improvements faster than competitors.
U.S. FDA Requirements for PMS
Under 21 CFR Part 822 and other regulations, manufacturers must:
- Monitor adverse events — Medical Device Reporting (MDR) requirements mandate that certain incidents be reported to FDA within 5 to 30 days, depending on severity.
- Conduct post-approval studies — For high-risk devices, FDA may require structured follow-up studies.
- Maintain complaint files — Every complaint (even non-serious) must be documented and reviewed for potential reporting.
- Submit periodic reports — Summaries of adverse events, corrections, and recalls.
EU MDR PMS Requirements
- Post-Market Surveillance Plan — Mandatory for every device, detailing data collection, analysis, and reporting methods.
- Periodic Safety Update Reports (PSUR) — Required for Class IIa devices and above, summarizing safety and performance.
- Post-Market Clinical Follow-Up (PMCF) — Gathering long-term performance data, especially for new or high-risk devices.
Recall Management
Even with the best design and manufacturing, recalls happen.
How you manage them can mean the difference between a short-term hit and a permanent reputation loss.
Types of Recalls
- Class I Recall — Reasonable chance of serious injury or death (most severe).
- Class II Recall — May cause temporary health problems or pose a slight threat.
- Class III Recall — Unlikely to cause harm but violates labeling/manufacturing regulations.
Best Practices for Handling Recalls
- Act Fast — Immediate internal investigation once an issue is flagged.
- Be Transparent — Inform regulators, customers, and distributors promptly.
- Have a Written Recall Plan — Assign roles, responsibilities, and escalation pathways in advance.
- Follow-Up Reporting — Submit root cause analysis and corrective actions to regulatory authorities.
Ongoing Compliance Obligations
- Quality System Audits — Internal and external audits to verify ongoing compliance with ISO 13485 or FDA QSR.
- Labeling & IFU Updates — Reflect new risk information or usage instructions.
- Field Safety Notices — Required in the EU for specific device safety alerts.
- Distributor Oversight — Ensuring downstream partners also follow compliance rules.
Leveraging PMS for Growth
Most companies treat post-market surveillance as a cost center — but smart CMOs and CEOs turn it into a competitive edge:
- Early Differentiation — “We don’t just meet minimum safety standards; we exceed them.”
- Product Roadmap Input — PMS data can guide upgrades and next-gen designs.
- Regulatory Favorability — Strong PMS systems can lead to faster future approvals.
💡 Pro Tip for Device Founders: Build PMS into your business model from day one. Treat every feedback channel — from clinical partners to end-users — as part of your quality loop.
📍 Next Up: Section 15 — Marketing & Sales Strategy for FDA-Registered Devices — where we’ll dive into compliant go-to-market planning, messaging, and scaling without running afoul of FDA advertising rules.
Do you want me to go ahead and push straight into Section 15 now so we keep momentum toward your 8,000-word target?
You said:
Next
ChatGPT said:
Here’s Section 15.
15. Marketing & Sales Strategy for FDA-Registered Devices
Launching an FDA-registered device means your marketing and sales strategy must walk a fine line — persuasive enough to drive adoption, compliant enough to pass regulatory scrutiny.
The mistake many founders make is treating device marketing like any other product launch.
That’s a fast track to FDA warning letters, reputational damage, and even civil penalties.
Understanding FDA Rules for Marketing
The FDA regulates labeling, promotional materials, and advertising for medical devices under the Federal Food, Drug, and Cosmetic Act (FD&C Act) and 21 CFR Part 801.
Key rules:
- Truthful and not misleading — All claims must be backed by valid evidence.
- Intended Use — You can’t promote uses not included in your 510(k) clearance or PMA approval.
- Balanced Risk-Benefit — Must present benefits alongside risks and limitations.
- Consistency with Labeling — Promotional content must match the device’s approved Instructions for Use (IFU) and labeling.
📌 Example: If your device is cleared for reducing mild wrinkles, you cannot claim it “reverses aging” or “eliminates all wrinkles” without clinical evidence and clearance for that claim.
Compliant Marketing Channels
- Professional Journals & Trade Publications — Highly credible and regulator-friendly.
- Medical Conferences & CME Events — Education-focused rather than purely promotional.
- Peer-Reviewed Studies & Case Reports — Clinical validation builds trust with both providers and patients.
- SEO & Content Marketing — Educational blogs, white papers, and guides that inform rather than “sell” aggressively.
What to Avoid
- Unsubstantiated Superlatives — “Best in the world,” “100% safe,” etc.
- Patient Testimonials with Unapproved Claims — If a patient says it cured them of something outside your intended use, you can’t use that testimonial without qualification.
- Overly Aggressive DTC Ads — Especially for prescription-only devices, these get extra regulatory scrutiny.
Building a Compliant Go-to-Market Plan
- Define Target Segments
- Hospitals & Clinics
- Group Purchasing Organizations (GPOs)
- Direct-to-Consumer (if allowed)
- Telehealth & Digital Health Integrations
- Map the Buyer Journey
- Awareness — Educational content, conference booths, earned media.
- Consideration — Case studies, demos, ROI calculators.
- Decision — Compliance-approved sales decks, peer referrals, and pilot programs.
- Develop Core Messaging
- Anchor around FDA-cleared claims.
- Use benefit-risk framing for credibility.
- Include proof points (clinical data, trial outcomes, real-world evidence).
- Enable the Sales Team
- Provide compliant scripts and claim checklists.
- Train them on what they can and cannot say.
- Ensure all materials are approved through Regulatory Affairs.
Integrating KOL (Key Opinion Leader) Strategy
In the medical device space, trusted physicians and researchers are often the most persuasive advocates.
- Engage early in product development for feedback.
- Involve in clinical trials to create authentic champions.
- Feature KOL-authored papers in marketing — with proper disclosure.
Digital Marketing in the Device Space
Even with strict compliance rules, digital marketing can be powerful:
- Webinars for continuing medical education (CME).
- SEO targeting for procedural terms your device supports.
- LinkedIn campaigns targeting procurement managers or physicians.
- Retargeting ads — but only with pre-approved copy.
Measuring Marketing ROI
FDA-registered device marketing success isn’t just about clicks or impressions:
- Sales Cycle Time — Often 6–18 months in B2B device sales.
- Adoption Rate — % of trained clinicians actively using your device.
- Reorder/Consumables Revenue — If your device has recurring components.
- Regulatory Incident Rate — Fewer compliance issues = healthier long-term growth.
💡 Pro Tip for Device CMOs: In the med device world, compliance is part of the brand. A marketing campaign that works but triggers a warning letter isn’t a win — it’s a liability.
16. Scaling Globally: CE Marking, International Regulations, and Market Entry Strategies
Once your FDA-registered device gains traction in the U.S., the natural next step is international expansion.
But crossing borders means entering a patchwork of regulatory regimes — each with its own rules, technical documentation standards, and market dynamics.
Why Global Expansion Matters
- Market Size — The global medical device market is projected to exceed $800B by 2030, with significant growth in Asia-Pacific, Latin America, and the EU.
- Diversified Revenue — Mitigates risk from U.S. market fluctuations or reimbursement changes.
- Innovation Credibility — Devices with both FDA clearance and CE marking are often viewed as global benchmarks.
CE Marking for the European Union
The CE mark is your entry ticket into the EU market — it signals compliance with the EU Medical Device Regulation (MDR 2017/745).
Steps to Obtain CE Marking:
- Classify Your Device
- Class I: Low risk (e.g., non-invasive instruments)
- Class IIa/IIb: Medium risk (e.g., diagnostic tools, certain implants)
- Class III: High risk (e.g., life-supporting devices)
- Appoint an EU Authorized Representative
- Required if you have no EU presence.
- Compile Technical Documentation
- Design & manufacturing details
- Clinical evaluation reports
- Risk analysis & management plans
- Engage a Notified Body
- Independent organizations that assess conformity for higher-class devices.
- Conduct Post-Market Surveillance (PMS)
- Continuous reporting and vigilance for device performance and safety.
📌 Note: MDR compliance is more stringent than the old MDD — expect deeper clinical evidence requirements.
Other Key International Markets
Canada
- Regulated by Health Canada under the Medical Devices Regulations (SOR/98-282).
- Requires Medical Device Licence (MDL) for Class II–IV devices.
- ISO 13485 certification is mandatory.
Australia
- Overseen by the Therapeutic Goods Administration (TGA).
- Uses a risk-based classification system similar to the EU.
- Many FDA or CE-marked devices qualify for expedited listing.
Japan
- Regulated by the Pharmaceuticals and Medical Devices Agency (PMDA).
- Requires Marketing Authorization Holder (MAH) in Japan.
- Clinical data often needs localization to Japanese populations.
Middle East & Latin America
- Varying requirements — often accept FDA/CE documentation but may require additional testing or labeling in local languages.
Global Market Entry Strategies
- Sequential Expansion
- Start with markets that have regulatory reciprocity with the FDA or CE mark to reduce barriers.
- Distributor Partnerships
- Leverage local distributors for market knowledge and faster entry.
- Joint Ventures
- Especially in markets with localization rules or import restrictions (e.g., China, India).
- Licensing & Technology Transfer
- Allow local manufacturers to produce under your brand in compliance with your quality systems.
Localization Beyond Language
- Labeling & IFUs — Adapt to meet local regulatory language requirements.
- Clinical Validation — Some countries demand local trials or post-market studies.
- Cultural Fit — Adapt marketing materials for local norms and healthcare practices.
Common Pitfalls in Global Expansion
- Underestimating Timeframes — CE marking can take 12–18 months for higher-class devices.
- Ignoring Post-Market Obligations — Some countries require annual re-certification.
- Weak Distributor Vetting — Poor local partnerships can tank brand perception.
💡 Pro Tip for Global Device CEOs: Don’t treat international markets as “copy-paste” versions of your U.S. strategy. Invest in local regulatory, clinical, and cultural expertise — it pays off in speed to market and adoption.
17. Post-Market Surveillance (PMS) & Compliance Maintenance
Getting your FDA registration or clearance isn’t the finish line — it’s the starting gun.
The real challenge begins after launch, when you must monitor, maintain, and document the safety, effectiveness, and quality of your device over its entire lifecycle.
Why PMS Matters
- Regulatory Requirement — Both FDA and international regulators mandate ongoing surveillance.
- Risk Management — Early detection of issues prevents costly recalls and litigation.
- Market Trust — Proactive monitoring reassures customers, payers, and partners.
U.S. FDA Post-Market Requirements
1. Medical Device Reporting (MDR)
- Timeline: Manufacturers must report device-related deaths or serious injuries within 30 calendar days of awareness.
- Form: FDA Form 3500A via the Electronic Submissions Gateway (ESG).
2. Corrections & Removals
- Any product correction or removal that reduces health risk must be reported to the FDA under 21 CFR Part 806.
3. Quality System Regulation (QSR)
- Maintain ongoing compliance with 21 CFR Part 820, including CAPA (Corrective and Preventive Action) processes.
4. Recalls
- Voluntary or mandatory recalls must follow FDA’s recall guidelines.
- Public notifications are required in certain scenarios.
International PMS Requirements
EU MDR
- Periodic Safety Update Reports (PSUR) — Required annually (Class III & implantable devices) or every two years (Class IIa/IIb).
- Post-Market Clinical Follow-Up (PMCF) — Continuous collection of clinical data.
ISO 13485 Integration
- Align PMS processes with ISO 13485 quality management systems to maintain global certification.
Building a PMS System
1. Data Sources
- Complaint logs
- Service reports
- Social media monitoring
- Device tracking and UDI data
- Clinical registry data
2. Feedback Loops
- Integrate findings into product design improvements.
- Feed safety signals into CAPA workflows.
3. Technology Tools
- Electronic QMS Platforms — Automate MDR, CAPA, and PMS documentation.
- AI Signal Detection — Use NLP tools to scan patient feedback for adverse event trends.
Key Performance Indicators for PMS
- Adverse Event Rate — Events per 1,000 units sold.
- Time to Resolution — Average days to close CAPA cases.
- Recall Frequency — Number of recalls per year.
- Regulatory Audit Findings — Number of observations in FDA 483s or NB audits.
Case Study: Proactive PMS Avoids Crisis
A Class II orthopedic device manufacturer implemented AI-driven monitoring that flagged an unusual spike in customer service calls mentioning “joint noise.”
Investigation revealed a minor design flaw in a hinge component.
The company:
- Issued a targeted field correction (not a full recall)
- Updated manufacturing specs
- Filed required MDRs
Result: No patient harm, zero lawsuits, and avoided brand-damaging headlines.
Common PMS Pitfalls
- Treating surveillance as a checkbox exercise.
- Poor integration of complaint handling into quality systems.
- Delayed reporting due to manual tracking.
💡 Pro Tip for Device CEOs: Post-market vigilance is a growth tool, not just a compliance chore. The faster you detect and address issues, the stronger your brand’s reputation and regulatory standing.
18. Scaling Through Strategic Partnerships
Even the most innovative FDA-registered device can stall if distribution, adoption, and market penetration lag behind.
Strategic partnerships are the fastest route to expanding reach, credibility, and sales — especially in regulated healthcare markets where trust and access are everything.
Why Partnerships Matter in MedTech
- Faster Market Access — Leverage partners’ established sales channels.
- Credibility Boost — Aligning with trusted healthcare brands accelerates adoption.
- Shared Risk & Cost — Reduce marketing and distribution expenses.
- Regulatory Leverage — Partners familiar with compliance can streamline your processes.
Types of Strategic Partnerships
1. Distributor Agreements
- Work with regional or global distributors who already serve your target market.
- Pros: Immediate access to existing customers.
- Cons: Lower margins; less control over messaging.
2. Group Purchasing Organizations (GPOs)
- Hospitals and clinics often purchase through GPOs.
- Securing a GPO contract can open hundreds of doors at once.
3. Co-Branding with Established Health Brands
- Joint marketing with a recognized brand in your category.
- Example: A wearable device partnering with a national fitness chain.
4. Hospital & Health System Alliances
- Pilot programs within major hospitals that become reference accounts.
- Clinical validation through case studies and peer-reviewed publications.
5. Technology Integrations
- Embed your device’s data into existing EHR platforms or patient apps.
- Enhances stickiness and adoption.
6. International Distribution Partnerships
- Work with companies familiar with CE Mark, Health Canada, TGA, or other markets.
- Avoids the pitfalls of going global alone.
Steps to Build High-Value Partnerships
Step 1: Identify the Leverage Point
- Who already sells to your ideal customer?
- Who has regulatory credibility in your target markets?
Step 2: Create a Partner Value Proposition
- Clearly define the ROI for your partner — more revenue, market differentiation, or competitive edge.
Step 3: Offer Market Exclusivity (Selective)
- Limited exclusivity can motivate partners to prioritize your product.
Step 4: Build a Co-Marketing Plan
- Joint PR, webinars, and case studies.
- Partner-specific sales enablement materials.
Step 5: Integrate Compliance Early
- Ensure partner agreements define regulatory and reporting responsibilities.
- Keep adverse event reporting processes aligned.
Key Metrics for Partnership Success
- Revenue Contribution — % of total sales from partners.
- Time to Market Entry — Months from agreement to first sale.
- Partner Engagement — Training attendance, co-marketing participation.
- Pipeline Growth — Leads or deals sourced through partners.
Case Study: Wearable Device Breaks Into Hospital Market
A startup with an FDA-registered remote monitoring wearable wanted to break into large hospital systems.
Instead of cold pitching, they partnered with a GPO already supplying 200+ hospitals.
Within 12 months:
- Closed contracts with 15 hospital systems.
- Secured a pilot program with the Mayo Clinic.
- Doubled valuation in the next funding round.
Common Partnership Pitfalls
- Relying on one “mega partner” instead of diversifying.
- Failing to train partner sales teams.
- No formal performance review or KPIs.
- Overpromising market exclusivity.
💡 Pro Tip for MedTech Founders: Partnerships are only as strong as your onboarding and support. Treat partner enablement like customer onboarding — the faster they see wins, the deeper the relationship.
19. Leveraging Digital Marketing & KOL Influence
In the FDA-registered medical device market, credibility and education are just as important as visibility.
Unlike consumer products, medical devices face longer adoption curves, multiple decision-makers, and strict advertising regulations — making digital marketing and Key Opinion Leader (KOL) advocacy a high-ROI combination.
Why Digital Marketing Matters in MedTech
- Educates Multiple Audiences — Clinicians, procurement teams, and patients often need different content.
- Shortens Sales Cycles — Well-informed buyers are faster to decide.
- Supports Compliance — Digital channels allow precise message control.
Core Digital Marketing Channels for FDA-Registered Devices
1. Content Marketing
- Whitepapers, clinical summaries, and explainer videos tailored to different stakeholders.
- Example: A 2-minute animated explainer for patients vs. a 10-page clinical efficacy summary for hospital boards.
2. SEO (Search Engine Optimization)
- Target condition-specific and solution-focused keywords.
- Build authority pages on your own site to outrank non-compliant competitors.
3. LinkedIn Thought Leadership
- Founders and clinical directors posting regularly on industry developments.
- Build trust with C-suite decision-makers and clinicians.
4. Webinars & Virtual Demos
- Real-time product walkthroughs with live Q&A.
- Great for showcasing usability, compliance, and clinical results.
5. Paid Media
- LinkedIn Ads for B2B targeting of healthcare administrators.
- Google Ads targeting patient and clinician searches (with compliance review).
- Retargeting campaigns for site visitors who don’t convert immediately.
Key Opinion Leader (KOL) Influence
Why KOLs Move the Needle
- Clinicians trust peers over sales reps.
- Published endorsements and clinical presentations create ripple effects in adoption.
- KOLs often sit on advisory boards, influencing purchasing decisions.
Types of KOL Partnerships
- Clinical Advisors — Guiding development and serving as public advocates.
- Conference Speakers — Presenting your device at industry events.
- Research Partners — Leading clinical trials and publishing results.
Building a KOL Program
- Identify Relevant Experts
- Specialists in your device’s therapeutic area.
- Clinicians already using similar tech.
- Create a Mutual Value Exchange
- Offer research opportunities, speaking engagements, or advisory fees.
- Integrate KOLs Into Marketing
- Case study videos.
- Guest-authored articles.
- Social media Q&A sessions.
- Measure Impact
- Track leads, media mentions, and adoption linked to KOL activity.
Case Study: Orthopedic Device Gains National Attention
An FDA-registered orthopedic implant manufacturer partnered with three leading surgeons to publish clinical results in a top peer-reviewed journal.
They amplified the study through:
- LinkedIn posts from the surgeons.
- Sponsored webinars with 500+ attendees.
- Conference presentations at AAOS.
Results in 9 months:
- Inbound distributor requests from 7 new states.
- 42% increase in sales pipeline.
- Surge in RFP invitations from major hospital systems.
Compliance Considerations
- All marketing claims must align with your device’s FDA-cleared labeling.
- Avoid implying unapproved uses or outcomes.
- KOL content must disclose financial relationships if present.
💡 Pro Tip for MedTech Marketers: Pair KOL advocacy with consistent digital content. KOLs open doors; your marketing keeps them open by nurturing every decision-maker in the buying process.
20. Post-Market Surveillance & Ongoing Compliance
Getting your medical device FDA-registered is a milestone — but it’s not the finish line.
The FDA and global regulatory bodies require continuous monitoring to ensure your device remains safe, effective, and compliant throughout its lifecycle.
What Is Post-Market Surveillance (PMS)?
Post-Market Surveillance refers to the ongoing process of collecting and analyzing data about a medical device after it has been released into the market.
It’s both a regulatory requirement and a business advantage.
Regulatory Drivers
- FDA Requirements — 21 CFR Part 803 (Medical Device Reporting) mandates that manufacturers report adverse events and device malfunctions.
- EU MDR Requirements — The European Union’s Medical Device Regulation has even stricter PMS obligations, including periodic safety update reports.
- ISO 13485 — Quality management standard requiring continuous monitoring.
Core Elements of a PMS Program
1. Adverse Event Reporting
- Identify and report any serious incidents within the FDA’s required timelines (usually 5–30 days).
- Maintain a centralized tracking system.
2. Complaint Handling
- Establish a documented process for receiving, reviewing, and resolving complaints.
- Categorize complaints by severity, frequency, and root cause.
3. Periodic Review Meetings
- Cross-functional teams (regulatory, quality, R&D, marketing) meet quarterly to review PMS data.
4. Field Actions & Recalls
- Have a written plan for corrective actions, including voluntary recalls if necessary.
- Communicate with regulators, customers, and distributors quickly.
5. Continuous Training
- Train sales reps, customer service, and clinical support teams on recognizing and reporting potential safety issues.
Business Advantages of Strong PMS
- Risk Reduction — Early detection prevents costly recalls and reputational damage.
- Regulatory Goodwill — A proactive PMS program can improve your standing with FDA auditors.
- Product Improvement — Real-world data informs R&D for next-gen device upgrades.
- Market Differentiation — Highlighting a robust safety monitoring program can build trust with buyers.
Leveraging PMS Data for Competitive Edge
- Marketing Proof Points — Share aggregated (non-identifiable) safety and performance stats in marketing materials.
- Investor Confidence — Demonstrates operational maturity and long-term viability.
- Partnership Opportunities — Pharma, insurance, and health system partners value documented safety performance.
Case Study: Using PMS to Drive Innovation
A wound care device manufacturer tracked customer feedback through a PMS portal.
They noticed a recurring complaint: difficulty applying the device with one hand.
Instead of ignoring it, they:
- Logged it in their PMS system.
- Tasked engineering to redesign the applicator.
- Relaunched with “single-handed application” as a feature.
Results:
- 35% reduction in user complaints.
- 22% increase in reorders.
- Faster adoption in home health settings.
Best Practices Checklist
- Centralize data collection in one PMS database.
- Assign a PMS coordinator.
- Review complaint logs monthly.
- Conduct root cause analysis for trends.
- Keep PMS documentation audit-ready.
💡 Pro Tip for MedTech Founders: Treat PMS as a marketing asset. Buyers — especially hospitals — trust companies that proactively monitor safety, even if they’ve never had a major incident.
21. Scaling Internationally: Expanding Beyond the U.S. Market
Once your medical device is FDA-registered, you’ve proven it meets one of the world’s toughest regulatory standards.
That gives you leverage — but it’s only the starting point if you want to go global.
Why International Expansion Matters
- Market Size — The U.S. accounts for ~40% of global medical device revenue. That means 60% is outside the U.S..
- Diversification — Multiple revenue streams protect against U.S. reimbursement or regulatory shifts.
- Investor Appeal — Investors value companies with multi-region presence because it signals scalability.
Key Regulatory Frameworks Beyond the FDA
1. CE Mark (European Union)
- Governed by the EU MDR (Medical Device Regulation).
- Requires conformity assessment by a Notified Body for most devices.
- CE Mark certifies that the product meets EU safety, health, and environmental requirements.
- Post-Brexit: Devices for the UK market require UKCA marking.
2. MDSAP (Medical Device Single Audit Program)
- Covers U.S., Canada, Brazil, Japan, and Australia.
- Allows a single regulatory audit to satisfy multiple market requirements.
3. TGA (Australia)
- Therapeutic Goods Administration regulates medical devices.
- Similar classification structure to the FDA’s, but with unique application requirements.
4. Health Canada
- Requires licensing based on device classification.
- Must comply with ISO 13485.
5. Other Notable Markets
- China (NMPA) — Often requires local clinical data.
- India (CDSCO) — Recently tightened medical device regulations; certain devices require local testing.
- Middle East (SFDA, MOHAP) — GCC countries often harmonize but require local representation.
Strategic Expansion Roadmap
- Market Prioritization
- Evaluate market size, competition, and reimbursement potential.
- Consider regulatory complexity vs. potential ROI.
- Gap Analysis
- Compare your FDA submission dossier with requirements in the target market.
- Identify missing testing, labeling, or clinical data.
- Local Representation
- Many countries require a locally registered agent or legal entity.
- Regulatory Submission
- Prepare documentation in the format required by the local authority.
- Translate labeling and IFUs (Instructions for Use) where necessary.
- Supply Chain Readiness
- Ensure you can fulfill orders within local regulatory and import rules.
- Post-Market Obligations
- Most countries require local adverse event reporting and vigilance systems.
Common Pitfalls in International Expansion
- Assuming FDA Clearance Equals Global Clearance — Most markets require separate approval processes.
- Underestimating Translation Costs — Regulatory and technical documentation translations can be expensive and time-consuming.
- Neglecting Local Reimbursement — Approval doesn’t guarantee payment; understand payer systems in each market.
Case Study: Phased Global Rollout
A mid-size diagnostics company:
- Phase 1 — Launched in Canada & Australia via MDSAP (shared audit cost).
- Phase 2 — Obtained CE Mark for EU entry.
- Phase 3 — Entered Brazil using MDSAP data to speed ANVISA approval.
Outcome: 3-year expansion added $42M in annual revenue while minimizing redundant testing.
Best Practices Checklist
- Conduct a regulatory landscape review for target countries.
- Leverage existing testing to reduce duplication.
- Budget for translations and local representation fees.
- Map reimbursement pathways before launch.
- Maintain region-specific PMS systems.
💡 Pro Tip for MedTech CEOs: Start your global strategy before your U.S. launch. Parallel applications can cut time to multi-market presence in half.
22. Leveraging Strategic Partnerships for Device Growth
In the medical device industry, going it alone can be slow, expensive, and risky.
Strategic partnerships allow you to accelerate market access, expand distribution, and amplify your credibility without shouldering the full burden of growth.
Why Strategic Partnerships Matter
- Market Access — Tap into established sales channels and customer bases.
- Shared Resources — Reduce R&D, marketing, and compliance costs.
- Credibility Boost — Association with respected brands increases trust.
- Regulatory Advantages — Local partners can help navigate country-specific compliance.
Types of Strategic Partnerships in MedTech
1. Distribution Partnerships
- What: Partner with companies that already sell complementary devices.
- Why: They already have relationships with hospitals, clinics, and procurement officers.
- Example: A wearable cardiac monitor company partnering with a medical equipment distributor serving 200+ hospitals.
2. Co-Development Partnerships
- What: Collaborate with another company to create a new device or integrate technologies.
- Why: Share R&D costs and combine expertise.
- Example: A surgical tools manufacturer teaming up with an AI company to embed diagnostic algorithms.
3. Licensing Agreements
- What: License your technology to another company for a specific region or application.
- Why: Allows monetization without building full sales operations in that market.
- Example: A U.S. wound care company licensing IP to a European manufacturer.
4. Academic & Clinical Partnerships
- What: Work with universities or research hospitals for studies, validation, and early adoption.
- Why: Clinical evidence and credibility from top institutions can make or break market acceptance.
- Example: Partnering with Mayo Clinic to conduct post-market clinical follow-up studies.
5. Technology Integration Partnerships
- What: Integrate your device into a larger platform or ecosystem.
- Why: Enhances your device’s value by making it part of a broader care pathway.
- Example: Integrating a glucose sensor with a telehealth diabetes management platform.
How to Identify the Right Partners
- Define Your Gaps
- Distribution? Technology? Clinical validation?
- Assess Cultural Fit
- Does the partner’s mission and style align with yours?
- Check Their Market Reach
- How large is their sales force? How deep are their customer relationships?
- Evaluate Financial Stability
- Avoid partnerships with financially unstable companies that could jeopardize supply or reputation.
- Review Regulatory History
- Ensure they have no history of compliance violations.
Structuring the Partnership
- Clear Scope of Work — Define responsibilities, timelines, and performance metrics.
- Exclusivity Clauses — Consider regional or product-line exclusivity carefully.
- Revenue Sharing & Margins — Align incentives for both parties.
- IP Ownership & Rights — Clarify who owns what in co-development scenarios.
- Exit Strategy — Define conditions under which either party can end the agreement.
Case Study: Distribution Partnership Success
A startup manufacturing portable ultrasound devices:
- Partnered with a global diagnostic equipment distributor with a 60-country footprint.
- The distributor handled regulatory submissions in each market.
- Sales grew from $3M to $18M in 24 months.
Lesson: A strong partner can multiply reach and speed far faster than internal hiring.
Best Practices Checklist
- Map potential partners by type (distribution, tech, clinical).
- Run due diligence on financials and compliance.
- Start with a pilot program before full rollout.
- Use data to monitor performance.
- Keep communication frequent and transparent.
💡 Pro Tip for MedTech Founders: Partnerships aren’t just for scale — they’re for positioning. Being tied to the right brand can shift you from “new player” to “market leader” in perception.
23. Post-Market Surveillance and Continuous Improvement
Launching an FDA-registered medical device is not the end of the journey — it’s the start of a constant feedback, monitoring, and improvement cycle.
In today’s regulatory and competitive environment, post-market surveillance (PMS) is both a compliance requirement and a strategic growth lever.
Why Post-Market Surveillance Matters
- Regulatory Requirement — FDA, EU MDR, and other regulatory bodies require ongoing monitoring of safety and performance.
- Risk Management — Detect issues early to prevent recalls, lawsuits, and brand damage.
- Product Iteration — Real-world usage data often reveals opportunities for performance upgrades or new features.
- Market Differentiation — Demonstrating continuous improvement builds trust with clinicians and patients.
Core Components of Post-Market Surveillance
1. Complaint Handling Systems
- Maintain a formal process for recording, investigating, and resolving customer complaints.
- Ensure all employees know how to log and escalate issues.
- Example: If multiple surgeons report a handpiece overheating, this triggers a technical review and corrective action.
2. Adverse Event Reporting
- Monitor for any serious incidents or malfunctions that could lead to patient harm.
- Report to the FDA via the Medical Device Reporting (MDR) system within required timeframes (5–30 days depending on severity).
3. Post-Market Clinical Follow-Up (PMCF)
- Collect clinical data after launch to confirm long-term safety and performance.
- Often required in the EU under MDR, but also increasingly expected in U.S. market submissions for future product updates.
4. Trend Analysis
- Aggregate data from customer service, distributor reports, warranty claims, and service logs.
- Identify recurring patterns that indicate underlying issues.
5. CAPA (Corrective and Preventive Actions)
- A formalized process to investigate root causes, implement fixes, and prevent recurrence.
- FDA inspectors frequently check CAPA logs during audits.
Tools and Technology for PMS
- EHR/EMR Integration — Pull usage and outcome data directly from clinical systems.
- IoT Device Monitoring — Connected devices can send performance metrics automatically.
- AI-Driven Analytics — Use AI to detect anomalies earlier than manual review.
- Customer Feedback Platforms — Automated surveys post-procedure or post-delivery.
Continuous Improvement Loop
- Data Collection — Real-world performance data, customer feedback, device logs.
- Analysis — Identify trends, anomalies, and improvement opportunities.
- Action — Implement design changes, software updates, or manufacturing process improvements.
- Verification — Confirm that changes resolve the issue without introducing new risks.
- Documentation — Maintain detailed records for audits and future submissions.
Case Study: Improving Reliability Through PMS
A Class II orthopedic device manufacturer noticed a 2% increase in warranty claims for locking mechanisms.
Post-market investigation revealed that certain sterilization cycles degraded a small plastic component faster than expected.
The company:
- Switched to a higher-grade polymer.
- Updated sterilization guidelines for hospitals.
- Reduced warranty claims by 85% within six months.
Lesson: PMS not only prevents regulatory headaches — it can cut costs and boost product reputation.
Global Perspective
- U.S. — MDR reports, Quality System Regulation (QSR) compliance.
- EU — Post-Market Clinical Follow-Up (PMCF) required for CE Mark under MDR.
- Canada — Mandatory incident reporting to Health Canada.
- Australia — Australian Register of Therapeutic Goods (ARTG) requires ongoing adverse event reporting.
Best Practices Checklist
- Have a written PMS plan before launch.
- Train your team on complaint handling and adverse event escalation.
- Use technology to automate monitoring wherever possible.
- Treat PMS data as a source of innovation, not just compliance.
- Share improvements publicly when appropriate to build trust.
💡 Pro Tip for MedTech Executives: PMS is not a cost center — it’s an early-warning system and product development engine. Companies that treat it strategically consistently outperform in retention and market share.
24. Building an Exit Strategy from Day One
For many medical device founders and executives, the ultimate goal isn’t just a successful product launch — it’s positioning the business for acquisition, merger, or IPO.
The decisions you make before your first sale can determine whether your company commands a premium multiple… or struggles to attract buyers at all.
Why Plan for an Exit Early
- Strategic Alignment — Knowing your ideal buyer profile shapes product roadmap, partnerships, and regulatory pathways.
- Valuation Optimization — Investors and acquirers pay more for clean compliance histories, scalable processes, and strong IP.
- Risk Reduction — Early planning prevents last-minute scrambles to fix documentation gaps or regulatory issues.
- Negotiation Power — A prepared business is in a position to say no to lowball offers.
Key Drivers of Medical Device Acquisition Value
1. Regulatory Cleanliness
- FDA inspection history free of major observations.
- No outstanding warning letters or unresolved CAPAs.
- Well-documented Design History File (DHF) and Device Master Record (DMR).
2. Proven Commercial Traction
- Recurring revenue from consumables, software licenses, or service contracts.
- High customer retention rates and low churn.
- Multiple distribution channels (direct sales, GPO contracts, OEM deals).
3. Defensible Intellectual Property
- Issued patents covering core technology, not just design features.
- Freedom-to-operate analysis confirming no infringement risks.
- Trademark protection for brand and product names.
4. Scalable Operations
- Quality systems that can handle 5x–10x growth without major rework.
- Documented SOPs for manufacturing, training, and onboarding.
- Established supplier relationships with redundancy.
5. Strong Financial Performance
- Clear margins on both device and consumables.
- Healthy EBITDA or clear path to profitability.
- Clean, auditable financial records.
Exit Pathways
- Strategic Acquisition — Large MedTech firms buying to expand product portfolio or enter a new category.
- Private Equity Buyout — PE firms acquiring to scale operations and flip for higher valuation.
- Merger — Combining with a complementary company for market expansion.
- IPO — Suitable for companies with large, scalable markets and strong growth.
Designing Your Company for Sale
- Build With the Buyer in Mind — If a major orthopedic player is your target acquirer, align your design controls, reimbursement strategy, and product pipeline with their market.
- Create a Data Room Early — Keep all regulatory filings, clinical trial data, and financials organized in a secure, shareable repository.
- Lock Down Key Contracts — Secure long-term agreements with top customers, suppliers, and distributors.
- Demonstrate Growth Levers — Have a clear, documented plan for how the business can scale in new geographies or product lines.
Case Study: Selling at a Premium
A cardiology device startup built a Class II product with recurring consumable revenue.
From day one, they:
- Kept meticulous FDA compliance records.
- Filed multiple patents.
- Built a network of 150 hospital customers with 92% retention.
- Documented a pipeline of two follow-on products.
When they went to market, three strategic buyers bid, pushing the sale price 38% above initial valuations.
Lesson: Exit value is engineered, not accidental.
Red Flags That Kill Deals
- Unresolved FDA warning letters.
- Key supplier without a backup.
- Over-reliance on a single major customer.
- Poor IP protection or ongoing litigation.
- Sloppy financial reporting.
Best Practices Checklist
- Identify your ideal acquirer profile.
- Maintain clean regulatory and compliance history.
- Build recurring revenue streams wherever possible.
- Protect IP early and thoroughly.
- Keep financials audit-ready.
- Create a scalable operations framework.
💡 Pro Tip for MedTech Founders: Don’t wait until year five to “get ready” for an exit. Every decision you make in product design, regulatory strategy, and go-to-market positioning either adds to or subtracts from your future valuation.
📍 Final Takeaway: Whether your goal is a $50M acquisition or a $500M IPO, building your FDA-registered medical device company with an exit in mind from day one gives you leverage, speed, and maximum payoff when opportunity knocks.
FAQ
1) What’s the difference between FDA registered, cleared, and approved?
“Registered” lists your company/device with the FDA. “Cleared” (510(k)) shows substantial equivalence to a predicate. “Approved” (PMA) is the highest bar, based on clinical evidence for Class III devices.
2) Do I need FDA approval to sell a Class I device?
Most Class I devices don’t need premarket review, but you still must register your establishment, list the device, meet labeling/QMS rules, and follow any special controls.
3) How do I know my device class (I, II, or III)?
Check the FDA Product Classification database for devices with the same intended use/tech characteristics. If uncertain, submit a 513(g) request or use a regulatory consultant.
4) What is a predicate device in a 510(k)?
A legally marketed device with the same intended use and similar technological characteristics. You compare your testing/results to the predicate to show substantial equivalence.
5) When should I use De Novo instead of 510(k)?
Use De Novo if no suitable predicate exists but the device is low‑to‑moderate risk and can be controlled with general/special controls.
6) When is PMA required?
For most Class III, high‑risk or novel life‑supporting devices where clinical evidence is needed to prove safety and effectiveness.
7) How long does FDA clearance or approval take?
Typical 510(k) reviews target ~90 FDA days (often 4–9 months including Q&A). De Novo commonly takes 6–12 months. PMA can run 1–3+ years due to trials and inspections.
8) What does an FDA Pre‑Submission (Q‑Sub) do?
It lets you ask FDA targeted questions on indications, testing, and study design before you spend money on validation/clinical work—often saving months later.
9) What tests are usually required before submission?
Depends on risk and device type, but often includes bench/performance tests, biocompatibility (ISO 10993), electrical safety/EMC (e.g., IEC 60601), sterilization, and shelf‑life.
10) Do all Class II devices need clinical data?
Not always. Many 510(k)s rely on bench/performance data, but novel features or higher risk can trigger clinical requirements.
11) What is a DHF, DMR, and DHR?
DHF documents your design process; DMR contains the specs needed to build/maintain the device; DHR records how each lot/batch was actually built.
12) What is ISO 13485 and do I need it?
It’s the international QMS standard for medical devices. While FDA has its own QSR, ISO 13485 is often expected and required for many global markets.
13) What is CAPA and why does FDA care?
Corrective and Preventive Action is your systematic process to investigate issues, fix root causes, and prevent recurrence. It’s one of the most audited QMS elements.
14) Can I market off‑label uses if doctors ask?
No. Physicians may use devices off‑label, but manufacturers may not promote off‑label uses in labeling, ads, or sales materials.
15) What claims can I make in marketing?
Only those consistent with your cleared/approved indications and adequately substantiated. Avoid implied clinical benefits you haven’t validated.
16) What triggers an FDA deficiency letter?
Gaps in testing, unclear risk analysis, inconsistent labeling/IFU, or weak substantial equivalence rationale. A strong pre‑submission and internal audit reduce this risk.
17) What is Medical Device Reporting (MDR)?
Your obligation to report certain adverse events and malfunctions to FDA within specific timelines (e.g., 5–30 days depending on severity).
18) How do recalls work?
If a product poses risk or violates regulations, you may initiate a correction/removal. You must notify FDA promptly, investigate root cause, and document CAPA.
19) What is UDI and do I need it?
Unique Device Identification improves traceability and recall management. Most devices require UDI on labels/packaging and GUDID data submission.
20) How should I pick a contract manufacturer (CMO)?
Audit their QMS, review certifications (e.g., ISO 13485), check inspection history, verify process validation, and define responsibilities in quality and supply agreements.
21) What if my submission gets rejected (NSE/denied)?
Request a meeting to clarify deficiencies, address gaps (e.g., additional testing/label revisions), and evaluate the right pathway (e.g., De Novo vs. 510(k)).
22) How do I plan clinical trials for devices?
Align endpoints with indications, follow GCP/ISO 14155, secure IRB approval, and obtain IDE for significant‑risk devices. Consider interim analyses and site feasibility.
23) What’s the fastest path to first revenue?
If possible, a clear 510(k) route with a recent predicate, tight bench testing plan, early Q‑Sub feedback, and a ready QMS/shipping/labeling stack.
24) Can I launch in the EU with just FDA clearance?
No. You need CE Marking under EU MDR via a Notified Body (for most classes), plus technical documentation, PMS/PMCF plans, and UDI/EUDAMED entries.
25) Do I need different labeling for other countries?
Yes. Most markets require local language, symbols, and region‑specific instructions and vigilance contacts. Plan labeling localization early.
26) How should I budget for regulatory costs?
Plan for FDA user fees, testing, QMS/eQMS software, clinical (if needed), consultants, and ongoing PMS. Under‑budgeting here is a top cause of delay.
27) What metrics matter post‑launch?
Complaint rate, adverse events, CAPA cycle time, recall rate, on‑time delivery, lot yield, adoption rate, reorder/consumable revenue, and audit observations.
28) How do I use compliance as a marketing advantage?
Publish clear indications and evidence, show privacy/safety commitments, and highlight quality stats (e.g., complaint rates, field corrections) to build purchaser trust.
Disclaimer: The information provided in this guide is for general educational and informational purposes only. It is not intended as, and should not be construed as, legal or regulatory advice. Launching a medical device involves complex requirements that may vary based on jurisdiction, product classification, and intended use. You should consult with qualified legal counsel, regulatory experts, and relevant authorities before making any decisions or taking action. The author and publisher assume no liability for any errors, omissions, or consequences arising from the use of this material.




